Leeds, England/leeds460
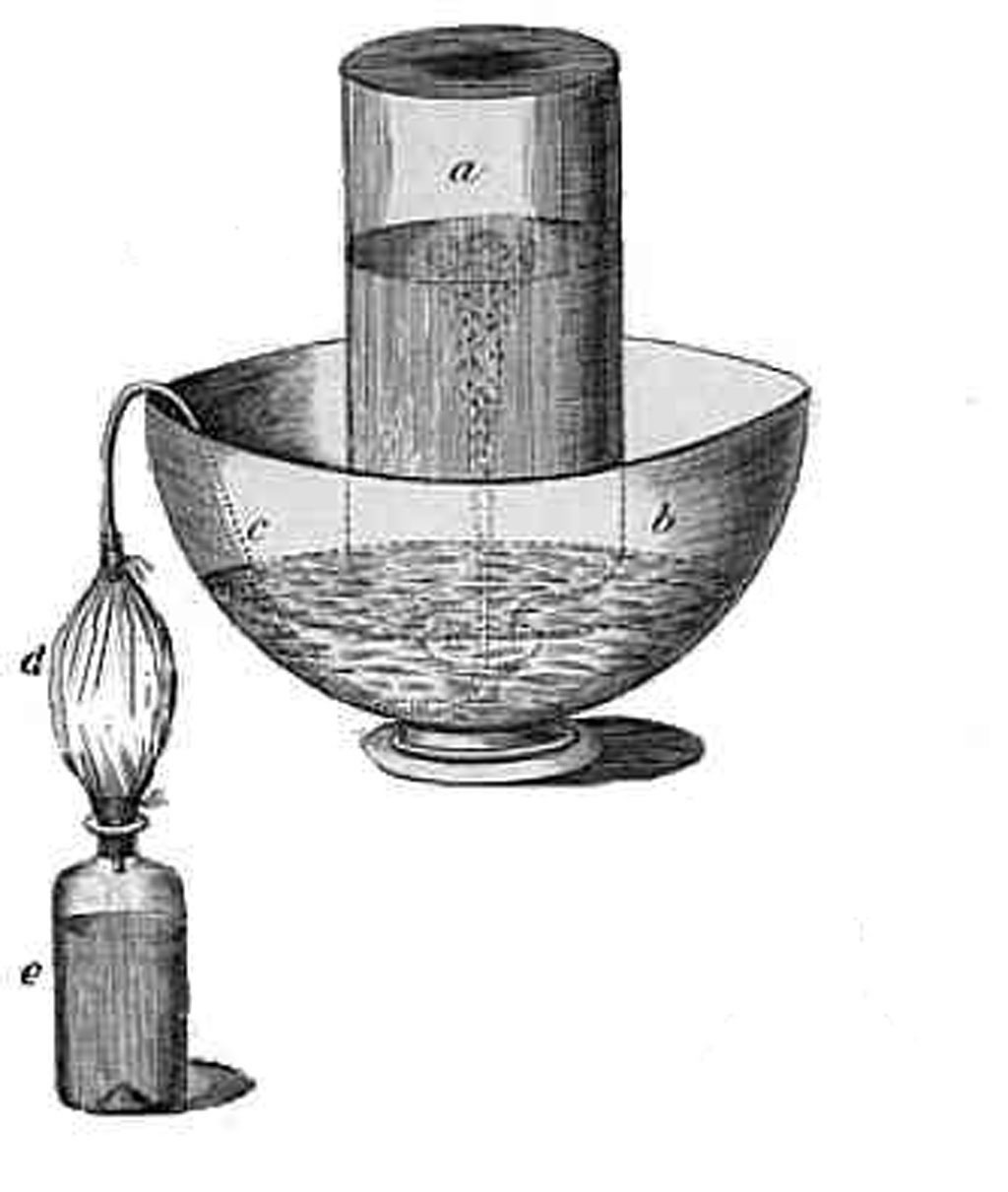
Previous | Home | NextEver the curious experimenter, Priestley soon devised a method of artificially producing carbon dioxide-impregnated water, which he called "Pyrmont water" after the German spa where this bubbly water was naturally produced {LINK: Bad Pyrmont 202}. In the bottle e to the left, oil of vitriol (sulfuric acid) and chalk (calcium carbonate) were mixed, producing a gas which passed through an intermediate bladder d (where it could be stored if desired) via a tube c to an inverted flask a trough of water b. Later, Priestley would investigate other gases, discovering that since some gases e.g., ammonia, sulfur dioxide) were soluble in water, a more suitable liquid was mercury. Thus was born "pneumatic chemistry" of which Priestley was the recognized master. At Leeds Priestley was able to discover and characterize "nitrous air" (nitrogen oxide, NO, prepared by reacting nitric acid and pyrites, metal sulfides), "acid air" (hydrogen chloride, HCl, directly from hydrochloric acid solution).